Aluminum production and its effect on climate change
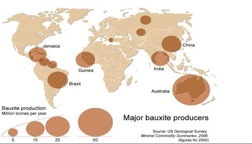
Bauxite is a mineral of enormous worldwide significance economically and ecologically. The term bauxite actually refers to any ore or mixture of minerals that is rich in the oxide that is formed from aluminous rocks. These minerals are gibbsite (Al(OH)3), diaspore (AlO(OH)), and boehmite (AlO(OH)). Because it is a mixture of minerals, bauxite itself is a rock, not a mineral. Bauxite forms when silica in aluminum-bearing rocks (that is, rocks with a high content of the mineral feldspar) is washed away (leached). This weathering process occurs in tropical and subtropical weathering climates. Most bauxite contains about 40-60% alumina. Deposits are found all over the world, from Spain through Southern France, Italy, Austria, Hungary and Greece, (providing the base for the European aluminum industry), with even larger deposits in subtropical and tropical regions of Africa, West Indies, South America and Australia. Bauxite is refined first into aluminum oxide trihydrate (alumina) and they electrolytically reduced into metallic aluminum. It takes two to three tones of bauxite to produce one ton of alumina and two tones of alumina to produce one ton of aluminum metal.
Bauxite deposits are generally extracted by open cast mining from strata typically 4 to 6 meters thick, under a shallow covering of topsoil and vegetation. During the preparation of a site the surface soil is removed, and then the next one to two meters of bauxite is drilled and blasted for extraction. Once the ore has been broken, it is loaded onto haul trucks by excavators or front-end loaders and transported to primary crushers at the mines. Unlike the base metal ores, bauxite does not require complex processing because most of the bauxite mined is of an acceptable grade or can be improved by a relatively simple and inexpensive process of removing clay.
In ore mining, the environmental effects are highly site specific, but, on the whole, the main impact comes from clearing the vegetation. One major environmental problem caused by the industry is the disposal of the tailings, which form an alkaline mud. The original procedure that was used to dispose of the red mud was to pump material into mined-out ore bodies and dyked valleys. According to the Trade and Environment database, readings obtained from domestic water wells in the vicinity of Jamaican alumina refineries have indicated elevated levels of sodium and pH readings. Sodium is associated with higher incidences of hypertension and can also degrade the habitat of local flora and fauna. Also, the escape of caustic soda (which is used to extract alumina from raw bauxite) into the groundwater supply significantly increases sodium concentration of domestic well water mostly in the rural areas. Erosion within the mined areas can be rapid if the soil is not recovered and reforested. The removal of vegetation can then bring about loss of flora and fauna, destruction of wildlife habitats, a possible spread of plant disease, changes in weather conditions, dust, and a possible need for runoff water treatment. Also, bauxite mining severely affects the water retention capability of the soil. Due to the enlargement of the surface area after mining, and the extraction of much bauxite, the soil is less capable of retaining water.
The aluminum industry relies on the Bayer process to produce alumina from bauxite. It remains the most economic means of obtaining alumina, which in turn is vital for the production of aluminum metal. The aluminum-bearing minerals in bauxite – Gibbsite, Bohmite and Diaspore are selectively extracted from the insoluble components (mostly oxides) by dissolving them in a solution of sodium hydroxide (caustic soda):
Gibbsite: Al(OH)3 + Na+ + OH- ---> Al(OH)4- + Na+
Bohmite and Diaspore: AlO(OH) + Na+ + OH - + H2O ---> Al(OH)4- + Na+
Diagram of the Bayer Process
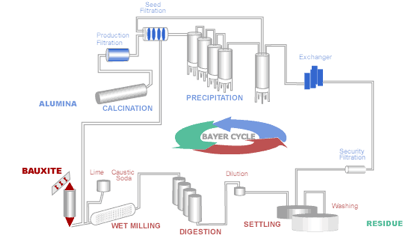
The ore is crushed and milled to reduce the particle size and make the minerals more available for extraction. It is then combined with the process liquor and sent in a slurry to a heated pressure digester. Conditions within the digester (concentration, temperature and pressure) are set according to the properties of the bauxite ore. After the extraction stage the insoluble bauxite residue must be separated from the Aluminum-containing liquor by a process known as settling. The liquor is purified as much as possible through filters before being transferred to the precipitators. The insoluble mud from the first settling stage is thickened and washed to recover the caustic soda, which is then recycled back into the main process. Crystalline aluminum trihydroxide (Gibbsite), conveniently named "hydrate", is then precipitated from the digestion liquor:
Al(OH)4- + Na+ ---> Al(OH)3 + Na+ + OH-
The "hydrate" crystals are then classified into size fractions and fed into a rotary or fluidized bed calcination kiln. Undersize particles are fed back into the precipitation stage. "Hydrate", is calcined to form alumina for the aluminum smelting process. In the calcination process water is driven off to form alumina:
2Al(OH)3 ---> Al2O3 + 3H2O
The calcination process must be carefully controlled since it dictates the properties of the final product.
In refining or alumina production, the environmental impact largely depends on the composition and quality of the ore and the processes used to extract bauxite. The principal hazards arise from the disposal or storage of bauxite-residue slurry (red mud), which is the main alkaline effluent from the alumina plants. Hence the red mud needs special precaution while disposing to avoid pollution of surface as well ground water resources. Airborne pollutants (dust and noxious chemicals) are another kind of hazard from stockpiles, mills, and calcination operations. The air pollutants are bauxite, lime, and alumina dust, SO2, NO2, dust from low-grade bauxite, and suspended vanadium pentoxide. The quality of SO2 pollution depends on its concentration in fuel oil, its specific form of consumption, and the ways power is supplied to the plant. Gas emissions not collected or insufficiently collected, especially in the case of SO2, can contaminate the workplace and the general environment and, in reacting with water, can produce acid rain.
The basis for all modern primary aluminum smelting plants is the Hall-Hroult Process, invented in 1886. Alumina is dissolved in an electrolytic bath of molten cryolite (sodium aluminum fluoride) within a large carbon or graphite lined steel container known as a "pot". An electric current is passed through the electrolyte at low voltage, but very high current, typically 150,000 amperes. The electric current flows between a carbon anode (positive), made of petroleum coke and pitch, and a cathode (negative), formed by the thick carbon or graphite lining of the pot. Molten aluminum is deposited at the bottom of the pot and is siphoned off periodically, taken to a holding furnace, often but not always blended to an alloy specification, cleaned and then generally cast.
There are environmental impacts associated with each stage of aluminum production, from extraction to processing. The major environmental impact of refining and smelting is greenhouse gas emissions. These gases result from both the electrical consumption of smelters and the byproducts of processing. The greenhouse gases resulting from primary production include perfluorocarbons (PFC), polycyclic aromatic hydrocarbon (PAH), fluoride, sulfur dioxide (S02), and carbon dioxide (CO2). Of these gases, PFC's resulting from the smelting process are the most potent to climate change. When the alumina ore content of the electrolytic bath falls below critical levels optimal for the above chemical reaction to take place, rapid voltage increases occur, termed "anode effects". During an anode effect, carbon from the anode and fluorine from the dissociated molten cryolite bath combine, producing CF4 and C2F6. These gases are emitted from the exhaust ducting system or other pathways from the cell (e.g., the hood of the cell).Sulfur dioxide and sodium fluoride are emitted from smelters and electrical plants. SO2 is one of the primary precursors of acid rain. CO2 emissions occur during smelting and result from the consumption of carbon anodes and from PFC emissions.
In a joint effort with the US Environmental Protection Agency, the aluminum industry cooperates in the Voluntary Aluminum Industrial Partnership to track, reduce, and report emissions and other environmental impacts related to primary aluminum production. Studies have shown that the global aluminum industry has made progress in reducing emissions of PFCs. The VAIP promotes the development and adoption of cost-effective PFC emissions reduction opportunities. The program is implemented through a Memorandum of Understanding between EPA and primary producer companies. VAIP represents 18 of the nations 19 smelters and 98% of U.S. production capacity. PFCs are emitted during anode effects that occur when the alumina ore content of the electrolytic process bath falls below critical levels optimal for the production of aluminum. During an anode effect, two PFCs - CF4 and C2F6 - are produced. The magnitude of PFC emissions for a given level of aluminum production depends on the frequency and duration of the anode effects. CF4 and C2F6 have global warming potentials (GWP) of 6,500 and 9,200 times as strong as CO2 and atmospheric lifetimes over 10,000 years. Thus, reducing emissions will have a significant environmental benefit for many future generations. In 2002, the U.S. primary aluminum industrys PFC emissions were equal to 1.42 million metric tons of carbon dioxide. This is the equivalent of emissions from approximately 1 million cars.
In a survey conducted by the International Aluminum Institute, on the Aluminum Industrys PFC reduction program, where survey questionnaires were sent out to 104 IAI facilities around the world, it was found that an overall 60% reduction in the specific emission rate for CF4 has occurred over the 1990 to 2000 time period. This is one of the few examples of where, the growth in global emissions of a greenhouse gas from an industry sector are actually in decline. The declining rate of PFC emissions is the result of the industry's efforts to reduce the frequency and also to some extent the duration of the anode effects in pot line cells.
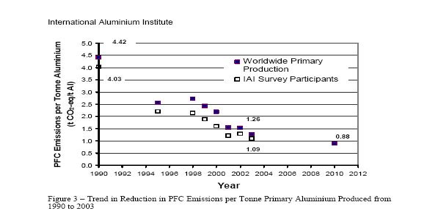
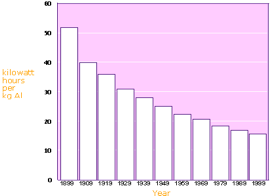
Another indirect hazard to the environment is due to the intensive consumption of electricity in the aluminum-smelting industry. The IEA (the International Energy Agency) has completed a new study Tracking Industrial Energy Efficiency and CO2- Emissions. On a global scale, the aluminum sector represents about 2.8% of world electricity demand (1.7EJ / year of electricity for smelters). It takes some 15.7 kWh of electricity to produce one kilogram of aluminum from alumina. Design and process improvements have progressively reduced this figure from about 21kWh in the 1950s. Globally smelters show a narrow electric efficiency range, compared to variations in energy efficiency in other industries. Therefore the energy efficiency potential in primary smelters is limited. The average efficiency has improved by 0.4% per year between 1980 and 2005. Industry is the only source for comprehensive data on aluminum materials flows through the economy. An important characteristic of the aluminum cycle is that the stock of aluminum in use is still rising rapidly. This limits the recycling potential significantly. According to the International Aluminum Institute, more than 55 per cent of the world's primary aluminum is produced using hydro-electric power which is clean, non polluting and renewable.
Hydro electric dams and their related aluminum smelters tend to be situated in remote areas, and provide economic activity where there would otherwise be none. The construction and the operation of these dams also pose a potential threat to the environment. Large areas have to be depopulated and flooded for dam building, changing the ecosystem of a whole area. This has potential negative effects on flora and fauna and even jeopardizes human health in the area. Most other aluminum smelters are located in areas where there is a natural surplus of energy for which there is insufficient economic local use.
Other environmental impacts of smelting include air pollution caused by fluoride emissions in the smelting process. These can have a strong effect on workers' health inside the plants (excessive intake can cause fluorosis and skeletal disorders), as well as on the surrounding flora and fauna. As molten cryolite (Na3AlF6) is used in the electrolysis of alumina, the fumes emitted from the cell have gaseous and particulate fluorides. Gaseous fluorides are also contained in the exhaust gas of the anode baking furnace and are emitted if unscrubbed. They can also be generated at a lower degree from the cast-house furnaces. Other fumes emitted by some types of cells are tar fumes (that contain suspected carcinogens) and SO2 (when petroleum coke with sulfur is used for the anodes); SO2 is also produced by anode-baking and cast-house furnaces, especially in plants using thermal power. At these last two stages, nitrogen oxides are also emitted. Dust is found at different stages in the production of aluminum. The main solid-waste problem is that of spent potliners, as cells have to be relined every 4 or 5 years. A typical aluminum smelter consists of around 300 pots. These will produce some 125,000 tonnes of aluminum annually. However, some of the latest generation of smelters are in the 350-400,000 tonne range. The linings can leach fluorides and cyanides to surface or groundwater when they are stored in the open air and in pits.
Aluminium is an extraordinarily versatile material. The range of forms it can take (castings, extrusions and tubes, sheet & plate, foil, powder, forgings etc) and variety of surface finishes available (coatings, anodizing, polishing etc) means it lends itself to a wide range of products, many of which we use every day of our lives. As aluminum is so lightweight this means that less energy needs to be used to move a vehicle made with aluminum than one made from a heavier metal, say steel. Although aluminum isn't the strongest of metals its alloys use other elements to pin dislocations in its structure to increase its strength. With trains, boats and cars aluminum is useful for this lightweight property (which gives fuel efficiency) but not essential, in planes however maintaining a relatively low weight is vital (in order to level the ground), and aluminum allows planes to have to this. In modern planes aluminum makes up 80% of their (unladen) weight, and a normal Boeing 747 contains about 75 000 kg of the metal. Its corrosion resistance is an advantage in transport (as well as packaging) as it makes painting planes unnecessary saving some hundreds of kilograms of further weight.
In a recent study by Nicola Stuber, published in the journal Nature, it is suggested that the contrails (artificial clouds that form around the tiny aerosol particles in airplane exhaust) overall impact on climate change is similar in scope to that of aircraft's carbon dioxide (CO2) emissions over a hundred-year period. Aircraft are believed to be responsible for 2 to 3 percent of human CO2 emissions. Like other high, thin clouds, contrails reflect sunlight back into space and cool the planet. However, they also trap energy in Earth's atmosphere and boost the warming effect, the study says. This warming effect is far greater for contrails left by night flights, Stuber added. "The solar cooling effect [wherein contrails reflect the sun's rays back into space] only happens during the day, when the sun is up," she explained. "During the night the greenhouse warming is no longer balanced, and that is why the contribution of nighttime flights is so large."
The UN's Intergovernmental Panel on Climate Change estimates that aviation causes 3.5 percent of global warming, and that the figure could raise to 15 percent by 2050. In 2000, a study conducted by the GAO (General Accounting Office) found that, in the United States, aviation emissions accounted for about three percent of the greenhouse gases and other emissions that contribute to the global warming phenomenon. While this percentage is small in relative terms-other transportation sources contribute 23 percent, and other industrial emissions account for 41 percent-aviation emissions are potentially significant for a number of reasons:
- Jet aircraft emissions are deposited directly into the upper atmosphere and some of them have a greater warming effect than gases emitted closer to the surface, such as automobile exhaust.
- The primary gas emitted by jet aircraft engines is carbon dioxide, which can survive in the atmosphere up to 100 years.
- Carbon dioxide combined with other exhaust gases and particulates emitted from jet engines could have two to four times as great an impact on the atmosphere as carbon dioxide emissions alone.
- The growing demand for jet air service is likely to generate more emissions that cannot be offset by reductions achieved through technological improvements alone.
The report recommended further research into the impact of jet exhaust on the global atmosphere to help guide the development of new aircraft engine technology. It also called upon governments to reduce emissions through improved air traffic control and regulatory and economic incentives.
In conclusion, aluminum processing and production does have a serious impact on the environment, and, as can be seen from the sources cited, it also contributes significantly to global climate change- not only via the processing of bauxite and alumina, but also, the products which aluminum is used, has similar effects. With increasing awareness of a sustainable earth, there are initiatives taken by the entire aluminum industry to reduce the effects of aluminum production on the environment. These include the EPA's Voluntary Aluminum Industrial Partnership (VAIP), which is leading the way. Other options including recycling of aluminum as recycled aluminum requires only 5 per cent of the energy required to make "new" aluminum. Blending recycled metal with new metal allows considerable energy savings, as well as the efficient use of process heat. There is no difference between primary and recycled aluminum in terms of quality or properties. The Department of Energy has also proposed "Aluminum Industry Technology Roadmap" in February 2003. The Roadmap presents industry-wide performance targets for energy, environment, and market share, and describes the research and development strategy for achieving those targets. One goal, pursued aggressively by industry, is the development of the advance cell technology, with inert anode and wettable cathode components. An advanced cell will not only reduce the energy requirements, but will also eliminate the carbon anode thereby removing the source of carbon for PFC generation. The aforementioned initiatives enable the Aluminum industry to be in a position to continue its global growth, while minimizing its environmental footprint. This sustainability challenge juggles the efficiency and capability of the industry to provide the populous with the luxury of aluminum products, while ensuring that the natural environment remains ecologically viable and able to meet the needs of future generations.
Bibliography
Kendall Holloway, Steven. The Aluminium Multinationals and the Bauxite Cartel. Palgrave Macmillan (February 1988)
Holecheck, Jerry. Natural Resources- Ecology, Economics and Policy. Prentice Hall. 2003
Lewis, T. Environmental Chemistry and Toxicology of Aluminum. CRC (September 1, 1989)
United States. General Accounting Office. Aviation and the Environment. February 2000. <http://www.gao.gov/new.items/rc00057.pdf>
Nicola Stuber, Piers Forster, Gaby Rdel & Keith Shine. The importance of the diurnal and annual cycle of air traffic for contrail radiative forcing Nature- International Weekly Journal of Science. 15 June 2006. <http://www.nature.com/nature/journal/v441/n7095/full/nature04877.html>
International Aluminum Institute. Report on the Aluminum Industrys Global Reduction of Perfluorocarbons gas emissions reduction program. Published 28th January 2005. <
http://www.world-aluminum.org/iai/publications/documents/aes_pfc_2003.pdf>
The Environmental Protection Agency. Voluntary Aluminum Industrial Partnership (VAIP). September 15th, 2006. <http://www.epa.gov/highgwp/aluminum-pfc/index.html>
Dr. Lee, James. The Trade and Environment database. <http://www.american.edu/TED/ted.htm>
U.S. Department of Energy. Aluminum Industry Technology Roadmap <http://www1.eere.energy.gov/industry/aluminum/pdfs/al_roadmap.pdf>